Automate IDMP Data Standardization and Unify Your Product Data Across All Systems
The ACCURIDS IDMP Fabric vs. Adapting Your Existing Systems
The Capabilities of the IDMP Data Standardization Fabric.
One Golden Record for Every Product
Empower Your Business Experts to Own Data Quality
Achieve Compliance at Radical Speed
.avif)
From Global Compliance Mandate to Strategic Asset
The Features That Power Your IDMP Solution
Automate End-to-End Product Data Standardization.
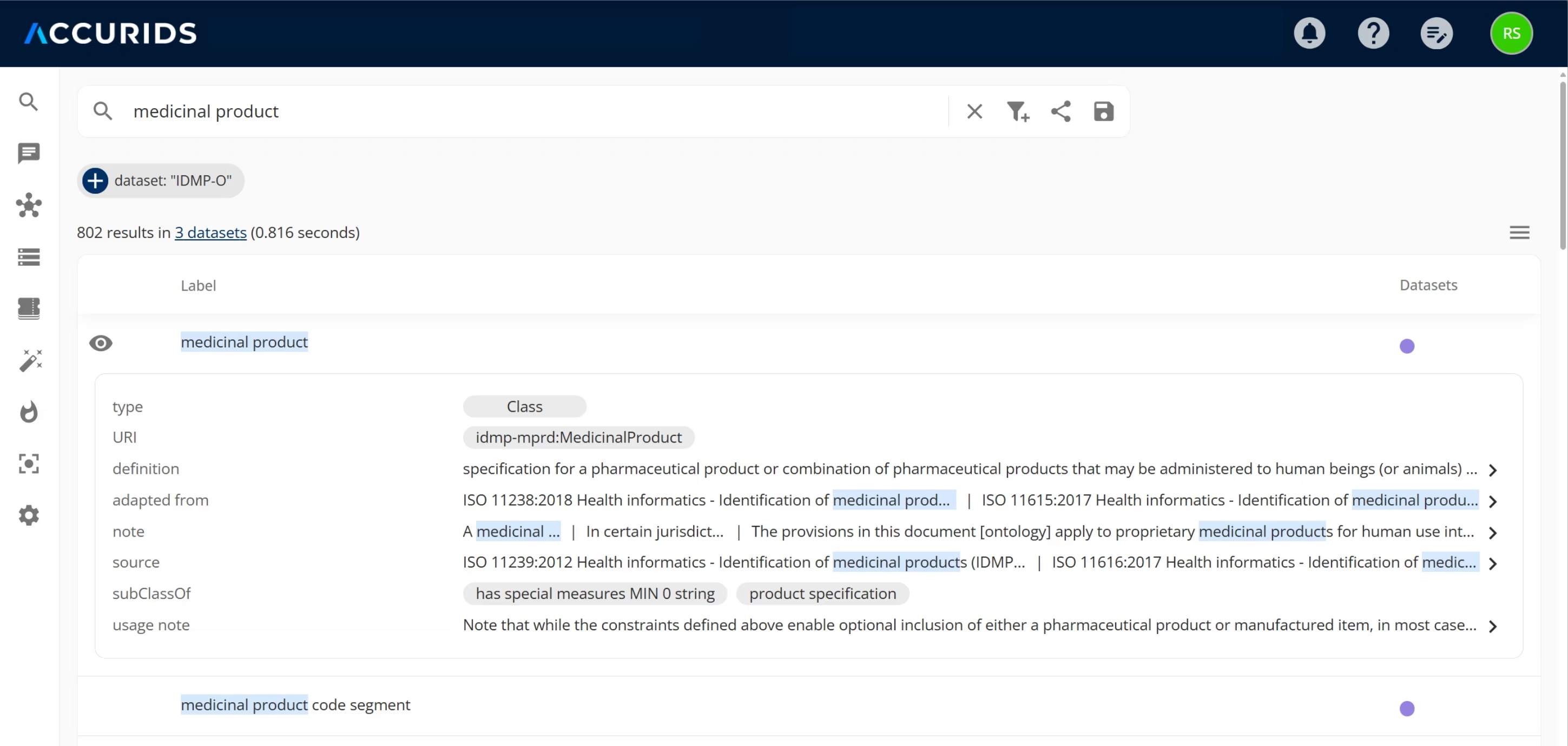
Unify Data from All Sources Without Replacing Your Systems.
Connect and Query Your Data Based on Relationships, Not Tables.
Move from Reactive Fire-Fighting to Proactive Governance.
Get a Head Start on Your Compliance Project.
Provide a User-Friendly View for Your Non-Technical Experts.
From Unified Data to Compliant Submission

Built on a Foundation of Unrivaled Expertise
Official Veeva Product Partner

1st Official IDMP Implementation Partner



Designed to Fit Your Existing IT Landscape

Security & Compliance
You Can Trust
Designed for GxP & 21 CFR Part 11
Flexible Deployment for Your Environment
Full Data Control & Isolation
Frequently asked questions
Engineered on the very IDMP Ontology we helped create, the Fabric offers a level of native compliance generic tools can't match. It connects seamlessly to your current systems, using context-aware AI to map and harmonize fragmented product data. The result is a unified, audit-ready backbone that ensures full IDMP compliance without the need for a 'rip-and-replace' strategy.
Our platform guarantees compliance through two core, distinct capabilities. First, the IDMP Standardization Engine automates the complex transformation, mapping, and matching of your diverse data into the correct format. Second, our entire platform has a Regulatory-First Design Built on IDMP-O, meaning its data model is based on the official IDMP Ontology that we co-developed. This ensures the highest possible precision and compliance certainty, making your data truly interoperable.
One of the biggest problems why IDMP projects fail is that teams attempt to force-fit generic tools to a complex, specific standard. We eliminate this risk entirely because our solution was built around IDMP and FAIR data from the core. As the official implementation partner that co-developed the IDMP Ontology with the Pistoia Alliance, our Fabric is the authoritative implementation of the standard itself, not another configuration attempt. This unique expertise is the ultimate guarantee of project success. In addtion our software is able to detect, monitor and resolve data quality across different sources - eliminating second biggest failure reason for IDMP projects.
Data quality is indeed a huge problem in pharma and lot's of data needs to be cleaned up. However, with ACCURIDS, you can leverage the Data Quality monitoring and resolution capabilities to ensure your team is working on the important quality issues in collaboration with Data Quality AI agents. Our platform is designed to end the "soul-crushing" grind of reactive, manual data cleaning. Our Unified, User-Friendly UI empowers your business experts and data stewards to perform proactive governance and curation in a single, central place. Combined with Automated, Continuous Data Quality Monitoring, this shifts your team from tedious fire-fighting to high-value, proactive data strategy, ensuring your data is always audit-ready.
Our automated engine accelerates the timeline from years to just months, delivering a tangible asset with minimal burden on your internal teams. To begin, we offer a "Data Quality Risk Assessment" as a low-threshold first step. This assessment ingests a sample of your data to provide a comprehensive quality score, immediately demonstrating the platform's value and giving you clear insights into your current data landscape.
News & Insights
Ready to See `the IDMP Data Standardization Fabric` in Action?