GPRAS 2025 Insights: The "Second Wave" of IDMP and the Future of Regulated Product Data
The Global Pharmaceutical Regulatory Affairs Summit (GPRAS) 2025, which took place in Berlin from October 21-23, once again brought the regulatory community together. After three days of productive discussions, our CEO Heiner Oberkampf's conclusion was clear: it remains "Europe’s best conference for the pharmaceutical regulatory community."
For ACCURIDS, GPRAS was a resounding success. At the center of all discussions was a clear theme: The "second wave" of IDMP has arrived. The focus is no longer just on compliance, but on building the trusted data foundation that AI and true collaboration truly depend on.
Key Takeaways
The conversations at our booth (#5) and in the sessions highlighted several clear industry trends:
- Data Roles in Regulatory are Emerging: Finally! While late, this is a critical step toward lasting data governance within regulatory departments.
- The IDMP Ontology (IDMP-O) is Key: This was reinforced by discussions and reports from the recent ISO meeting in Toronto. A key confirmation from that meeting was: “The IDMP Ontology can help to ensure consistency and interoperability across IDMP implementations.”
- EMA's Impressive Progress: The EMA’s Product Master Data Service (PMS) is evolving rapidly—from the new write API enabling automation to completed mappings with national authorities for critical medicines.
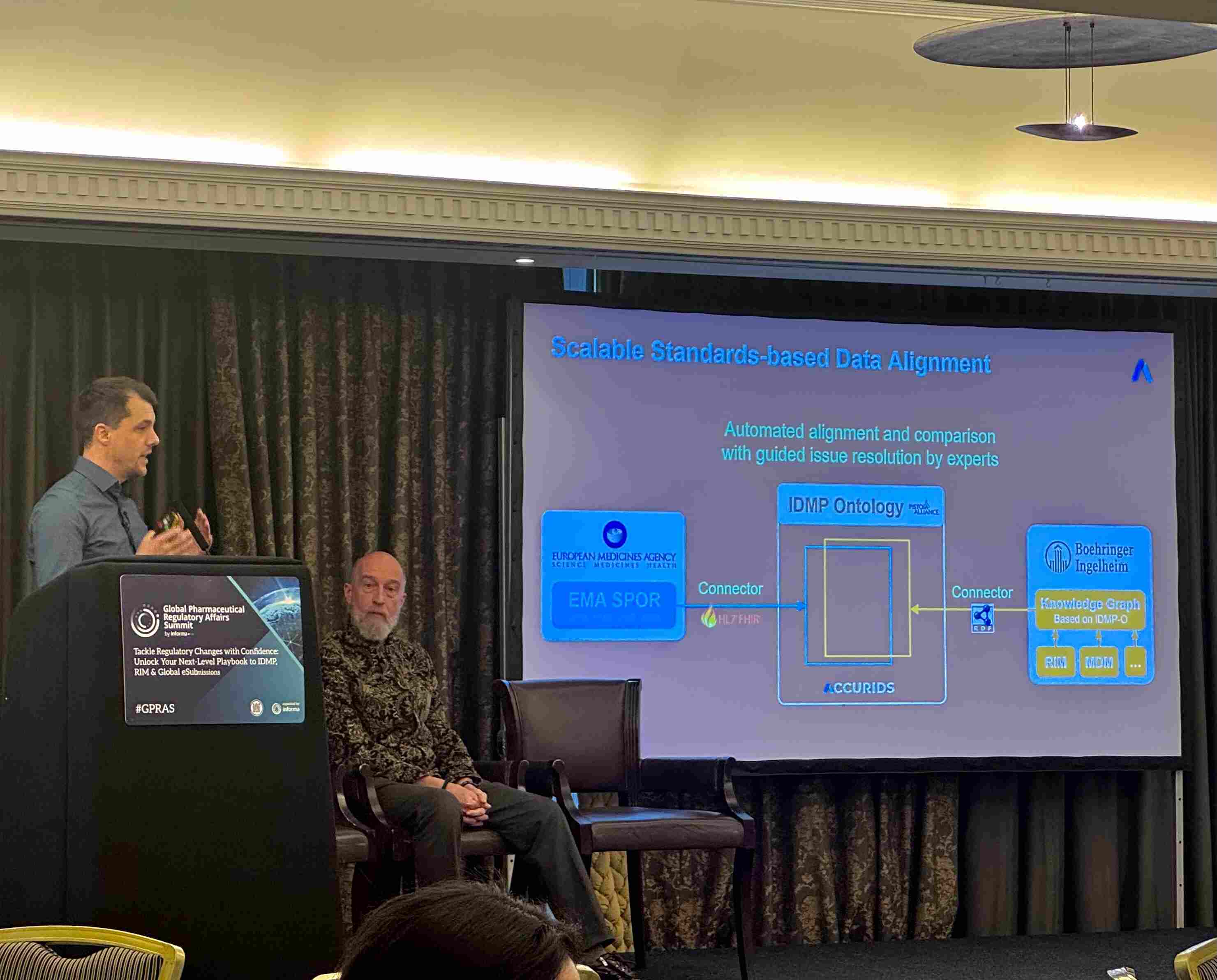
Joint Presentation with Boehringer Ingelheim
“Product Data Quality Across Sources – A Scalable Approach Aligning EMA PMS with Pharma RIM via the IDMP Ontology”
Our presentation demonstrated a pragmatic path for pharma companies to achieve data quality and harmonization at scale. Key points from the session included:
- Enhancing data quality through the use of innovative ontologies.
- Streamlining product management by integrating internal RIM systems with EMA PMS requirements.
- Driving efficiency across the entire product lifecycle by making structured, semantically rich product data actionable for regulatory, clinical, safety, and quality functions.

Celebrating the Veeva Product Partnership
Our booth was a fantastic hub for sharing our vision: a future where every critical decision in the life sciences industry is powered by clean, connected, and instantly accessible data.
A special moment during the event was connecting with the Veeva Systems team. As a proud Veeva Product Partner, we share a passion for innovation that makes a real difference. The discussions reinforced the importance of a connected ecosystem—especially in our focus areas of Regulatory Vault, Clinical Vault, Quality Vault, and Safety Vault.

Thank you to everyone for the inspiring conversations. We look forward to co-creating this next chapter of regulatory innovation.
Read more
From Fragmented Data to a Unified Backbone. In Months, Not Years.
.avif)

.png)



