Our purpose-built IDMP Product Data Standardization Fabric enables pharma to automate the complex process of unifying scattered data for regulatory compliance, eliminating the risk and cost of manually adapting generic systems to IDMP.

Without standardization, yesterdays data can't power tomorrow's breakthroughs
Product data in pharma is scattered across countless systems. Trying to force-fit these systems for IDMP compliance is a slow, error-prone, and labor-intensive process that creates critical bottlenecks.
High Effort &
Time Intensiveness
Jeopardized Compliance
& Blocked AI
Reactive Governance & Bottlenecks
A Purpose-Built IDMP Fabric that Creates a Trusted Product Data Backbone from Your Fragmented Data
Our IDMP Fabric combines an automated standardization & matching engine with the expert operationalization of the official IDMP-O standard to transform scattered data into a single, reliable asset at speed.
Creates a Future-Proof AI-Ready Data Foundation
Reduces Manual Effort and Expert Bottlenecks
Achieve Compliance in Months, Not Years
The Strategic Foundation for Data-Driven Leaders
"ACCURIDS has been a key implementation partner in the IDMP Ontology project, playing a pivotal role in building and championing the adoption of the IDMP Ontology within our pharmaceutical community. The team helped to transform the expansive vision of ISO IDMP standardization into reality with their expertise and semantic data standardization software and their practical implementations and collaborative demonstrations have been instrumental in the success of our project."

How the IDMP Product Data Fabric works
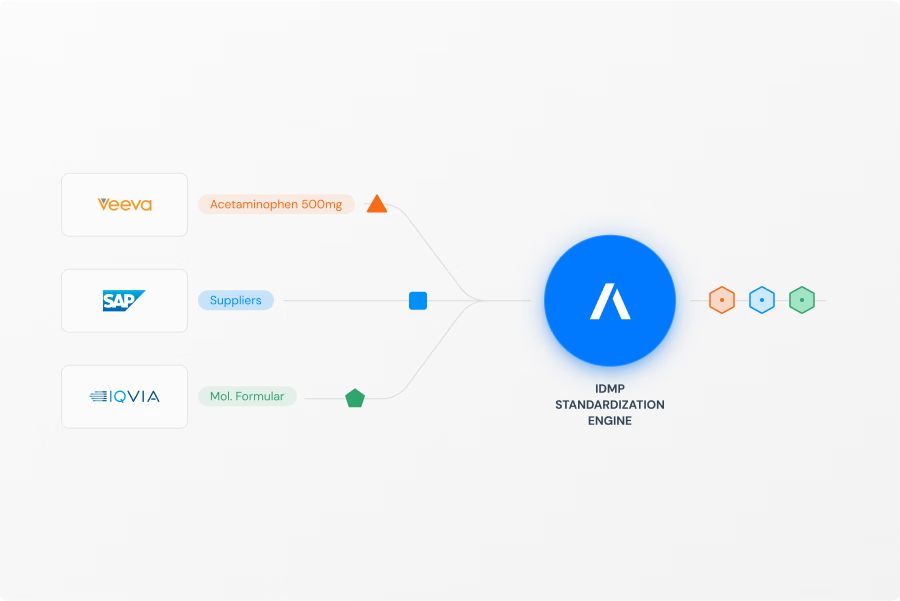
Connect & Standardize
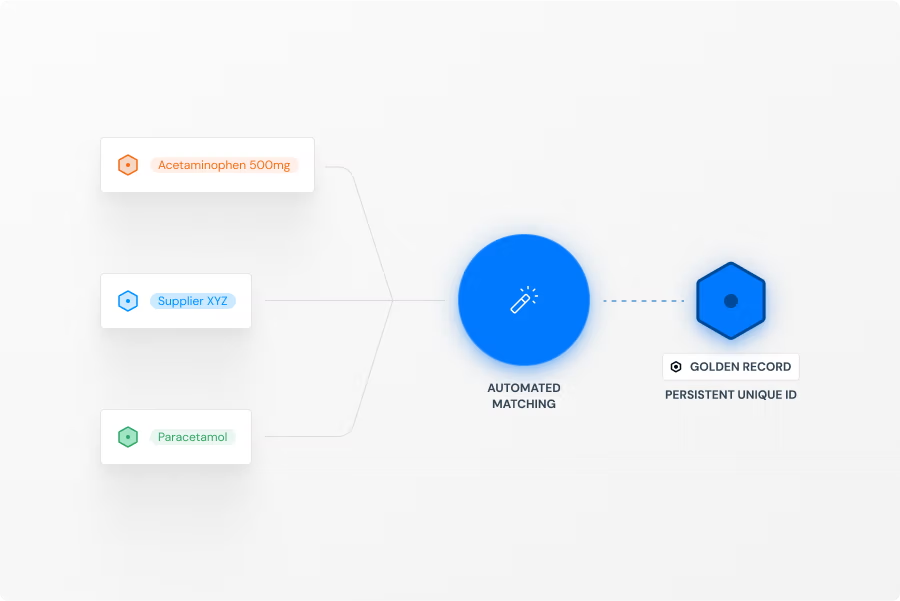
Match & Unify
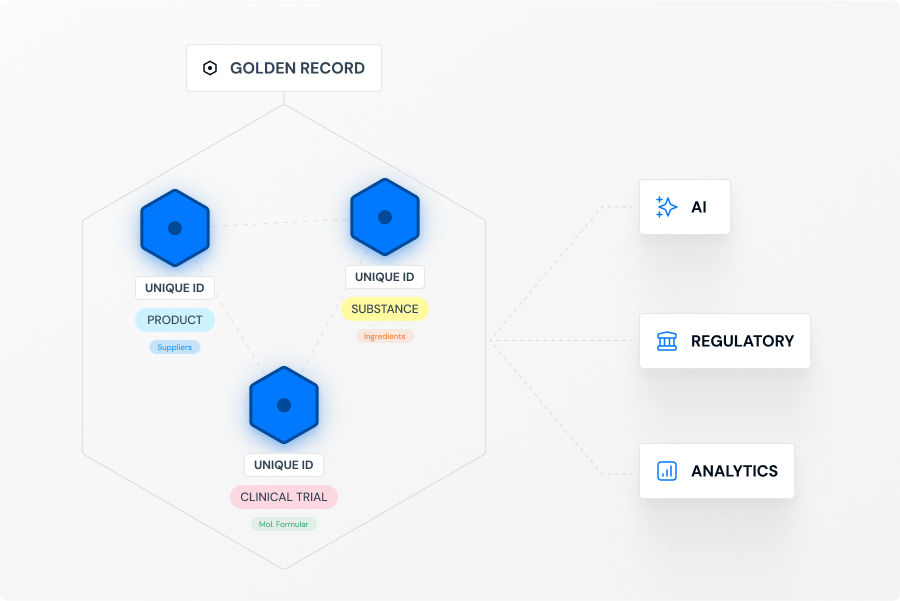
Access & Utilize
Built on a Foundation of Unrivaled Expertise
Official Veeva Product Partner

We Co-Developed the IDMP Ontology


How a Top 10 Pharma Company Built an IDMP-Aligned Knowledge Graph in Under 100 Days with ACCURIDS
Case Study
Security & Compliance
You Can Trust
Designed for GxP
&
21 CFR Part 11
Flexible Deployment for Your Environment
Full Data Control
& Isolation
Built to solve core data challenges of large and mid-sized pharma
IDMP Standardization Fabric
EMA PMS Data Alignment
Enterprise Data Registry
News & Insights
Frequently asked questions
Product data in pharmaceutical companies is typically scattered across countless systems like MDM, RIM, and ERP, and many different files using inconsistent terminology. This fragmentation makes adapting to new regulatory standards like IDMP an extremely slow, error-prone, and manual process. ACCURIDS solves this by transforming your scattered data into a single, reliable, and audit-ready asset, creating a trusted "Product Data Backbone" connected to the entire enterprise.
Adapting existing systems like RIM or MDM often means trying to force-fit a generic tool for a very specific and complex standard. This approach typically requires expensive, high-risk customization projects and heavy reliance on external consultants to make it work - and despite all that the result is a system standard not directly interoperable with other IDMP implementations. The ACCURIDS approach is fundamentally different. Our purpose-built "IDMP Product Data Standardization Fabric" leverages the ISO IDMP Ontology and HL7 FHIR as implementation standards and implements those as a non-invasive, intelligent layer that connects to your existing systems without replacing them. Think of it as a universal translator for your data. Instead of disruptive "rip-and-replace" projects , our fabric connects to your sources as they are, logically unifying the fragmented information into a single, cohesive, and audit-ready asset. This makes your product data immediately available and interoperable across the enterprise, without altering the underlying systems.
Data standardization projects often fail because organizations try to configure generic tools for a highly specific and complex standard. ACCURIDS eliminates this risk because our solution was purpose-built around the IDMP standard itself. We initiated the ISO IDMP Ontology project at Pistoia Alliance with innovative pharma companeis, co-developed it over the last 4 years and are the official implementation partner. As we already helped many organizations standardize their product data with the IDMP Ontology - our software, pre-standardized data assets and team provide the most effective way for you to implement IDMP within a few months, de-risk your project and ensure success.
Our platform follows a clear, automated three-step process. First, we Connect & Standardize, using our Standardization Engine to transform data from your existing systems into the uniform IDMP Ontology format. Second, we Match & Unify these records to eliminate redundancies and create single, complete "Golden Records". Finally, we Access & Utilize by connecting these records in our Knowledge Graph to create a unified "Product Data Backbone". This process is fine tuned with AI and a huge repository of public and private product data that allows to contextualize your product data and minimizes SME involvement to verify data quality and consistency. The resulting semantic data network is immediately available as a intelligent central source of truth for your critical processes, analytics, and AI initiatives.
IDMP is the entry point, but the core outcome is a long-term strategic asset: a trusted, enterprise-wide "Product Data Backbone". This unified data foundation is AI-ready and built to enable future strategic initiatives far beyond the initial compliance use case. By ensuring data is clean, contextualized, and interoperable, the backbone accelerates critical processes like "Time to Submission" and "Tech-Transfer," ultimately speeding up "Time-to-Peak-Sales" and optimizing the entire value chain.
From Fragmented Data to a Unified IDMP Backbone. In Months, Not Years.
.avif)


